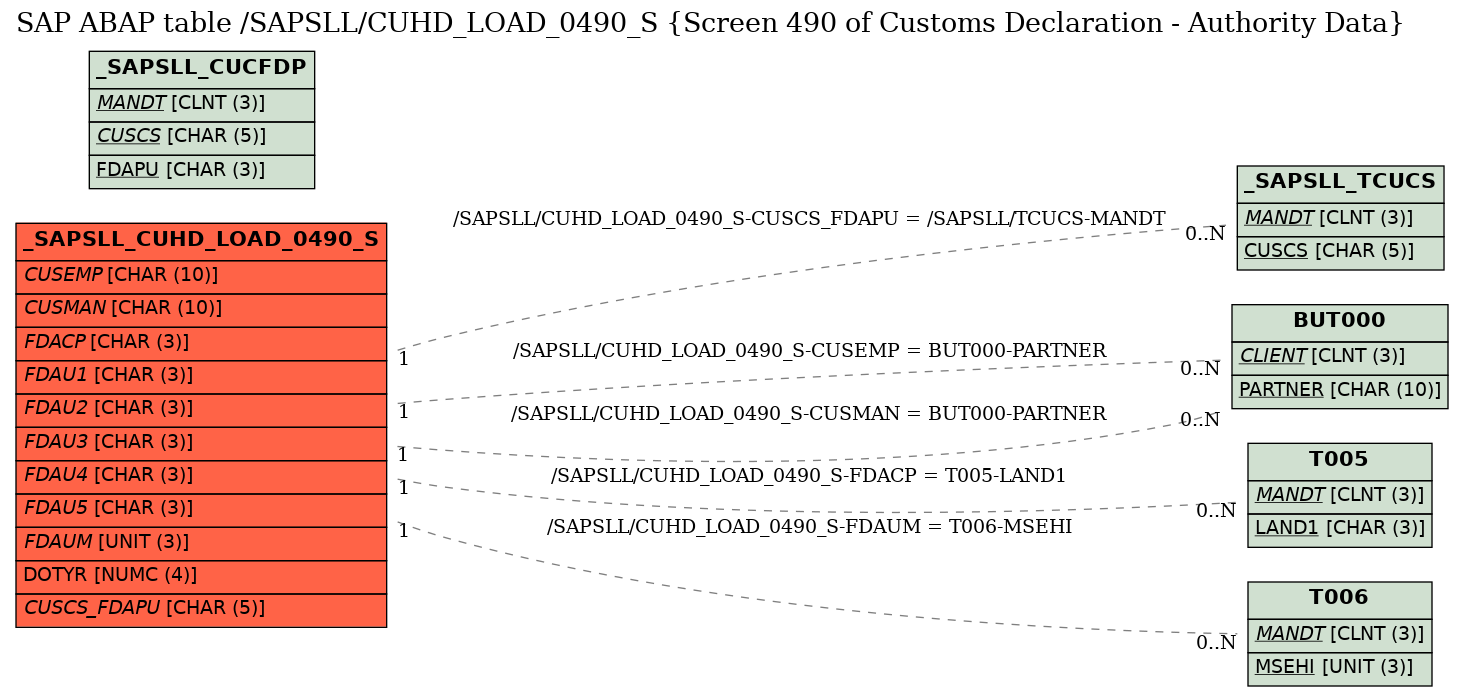
SAP ABAP Table /SAPSLL/CUHD_LOAD_0490_S (Screen 490 of Customs Declaration - Authority Data)
 Hierarchy
Hierarchy
☛
 SLL-LEG (Software Component) SLL-LEG 901: Add-On Installation
SLL-LEG (Software Component) SLL-LEG 901: Add-On Installation
⤷ SLL-LEG (Application Component) Global Trade Services
SLL-LEG (Application Component) Global Trade Services
⤷ /SAPSLL/CORE_LEGAL (Package) Legal Services: Core Functions
/SAPSLL/CORE_LEGAL (Package) Legal Services: Core Functions
⤷
⤷
 Basic Data
Basic Data
| Table Category | INTTAB | Structure |
| Structure | /SAPSLL/CUHD_LOAD_0490_S |
|
| Short Description | Screen 490 of Customs Declaration - Authority Data |
 Delivery and Maintenance
Delivery and Maintenance
| Pool/cluster | ||
| Delivery Class | ||
| Data Browser/Table View Maintenance | Display/Maintenance Allowed with Restrictions |
 Components
Components
| |
Field | Key | Data Element | Domain | Data Type |
Length | Decimal Places |
Short Description | Check table |
|---|---|---|---|---|---|---|---|---|---|
| 1 | |
/SAPSLL/LGREG | /SAPSLL/LGREG | CHAR | 5 | 0 | Legal Regulation | * | |
| 2 | |
/SAPSLL/CUSEMP | BU_PARTNER | CHAR | 10 | 0 | Consignee's Business Partner Number | BUT000 | |
| 3 | |
BU_DESCRIP_LONG | CHAR80 | CHAR | 80 | 0 | Description of a Business Partner | ||
| 4 | |
/SAPSLL/PAFCT | /SAPSLL/PAFCT | CHAR | 8 | 0 | Partner Function | * | |
| 5 | |
/SAPSLL/CUSMAN | BU_PARTNER | CHAR | 10 | 0 | Business Partner Number of Manufacturer | BUT000 | |
| 6 | |
BU_DESCRIP_LONG | CHAR80 | CHAR | 80 | 0 | Description of a Business Partner | ||
| 7 | |
/SAPSLL/PAFCT | /SAPSLL/PAFCT | CHAR | 8 | 0 | Partner Function | * | |
| 8 | |
0 | 0 | FDA Data of Customs Document | |||||
| 9 | |
0 | 0 | FDA Data of Customs Product | |||||
| 10 | |
/SAPSLL/FDACS | /SAPSLL/FDACS | CHAR | 1 | 0 | FDA Freight Storage Status | ||
| 11 | |
/SAPSLL/FDATN | /SAPSLL/TEXT40 | CHAR | 40 | 0 | FDA Brand Name | ||
| 12 | |
/SAPSLL/FDACP | LAND1 | CHAR | 3 | 0 | FDA Country of Manufacture | T005 | |
| 13 | |
0 | 0 | FDA Quantities | |||||
| 14 | |
/SAPSLL/FDAPQ | /SAPSLL/FDAPQ | INT4 | 10 | 0 | FDA Number of Packages | ||
| 15 | |
/SAPSLL/FDAPU | /SAPSLL/FDAPU | CHAR | 3 | 0 | FDA Package Type | /SAPSLL/CUCFDP | |
| 16 | |
/SAPSLL/FDAPQ | /SAPSLL/FDAPQ | INT4 | 10 | 0 | FDA Number of Packages | ||
| 17 | |
/SAPSLL/FDAPU | /SAPSLL/FDAPU | CHAR | 3 | 0 | FDA Package Type | /SAPSLL/CUCFDP | |
| 18 | |
/SAPSLL/FDAPQ | /SAPSLL/FDAPQ | INT4 | 10 | 0 | FDA Number of Packages | ||
| 19 | |
/SAPSLL/FDAPU | /SAPSLL/FDAPU | CHAR | 3 | 0 | FDA Package Type | /SAPSLL/CUCFDP | |
| 20 | |
/SAPSLL/FDAPQ | /SAPSLL/FDAPQ | INT4 | 10 | 0 | FDA Number of Packages | ||
| 21 | |
/SAPSLL/FDAPU | /SAPSLL/FDAPU | CHAR | 3 | 0 | FDA Package Type | /SAPSLL/CUCFDP | |
| 22 | |
/SAPSLL/FDAPQ | /SAPSLL/FDAPQ | INT4 | 10 | 0 | FDA Number of Packages | ||
| 23 | |
/SAPSLL/FDAPU | /SAPSLL/FDAPU | CHAR | 3 | 0 | FDA Package Type | /SAPSLL/CUCFDP | |
| 24 | |
/SAPSLL/FDAQU | /SAPSLL/DIMEN | QUAN | 19 | 3 | FDA Quantity | ||
| 25 | |
/SAPSLL/FDAUM | MEINS | UNIT | 3 | 0 | FDA Unit of Measure | T006 | |
| 26 | |
/SAPSLL/FDAPC | /SAPSLL/CCNGN | CHAR | 30 | 0 | FDA Product Code | ||
| 27 | |
/SAPSLL/GUID_16 | SYSUUID | RAW | 16 | 0 | Primary Key as GUID in 'RAW' Format | ||
| 28 | |
/SAPSLL/GUID_16 | SYSUUID | RAW | 16 | 0 | Primary Key as GUID in 'RAW' Format | ||
| 29 | |
0 | 0 | FCC Data of Customs Document | |||||
| 30 | |
0 | 0 | FCC Data of Customs Product | |||||
| 31 | |
/SAPSLL/FCCTN | /SAPSLL/TEXT40 | CHAR | 40 | 0 | FCC Brand Name | ||
| 32 | |
/SAPSLL/FCCCN | /SAPSLL/FCCCN | CHAR | 2 | 0 | FCC Import Condition | ||
| 33 | |
/SAPSLL/FCCCA | XFELD | CHAR | 1 | 0 | FCC Import Condition: Quantity Allowance | ||
| 34 | |
/SAPSLL/FCCID | /SAPSLL/TEXT20 | CHAR | 20 | 0 | FCC Identification | ||
| 35 | |
/SAPSLL/FCCMN | /SAPSLL/TEXT20 | CHAR | 20 | 0 | FCC Type Number | ||
| 36 | |
/SAPSLL/FCCWI | XFELD | CHAR | 1 | 0 | FCC Non-Publishing | ||
| 37 | |
0 | 0 | DOT Data of Customs Document | |||||
| 38 | |
0 | 0 | DOT Data of Customs Product | |||||
| 39 | |
/SAPSLL/DOTCC | /SAPSLL/DOTCC | CHAR | 1 | 0 | DOT Product Group | ||
| 40 | |
/SAPSLL/DOTTN | /SAPSLL/TEXT40 | CHAR | 40 | 0 | DOT Brand Name | ||
| 41 | |
/SAPSLL/DOTMO | /SAPSLL/TEXT20 | CHAR | 20 | 0 | DOT Model Name | ||
| 42 | |
/SAPSLL/DOTVE | /SAPSLL/TEXT20 | CHAR | 20 | 0 | DOT Number of Certificate of Suitability | ||
| 43 | |
/SAPSLL/DOTMV | /SAPSLL/TEXT20 | CHAR | 20 | 0 | DOT Manufacturer Name | ||
| 44 | |
/SAPSLL/DOTYR | KJAHR | NUMC | 4 | 0 | DOT Year | * | |
| 45 | |
/SAPSLL/VIDNO | /SAPSLL/TEXT30 | CHAR | 30 | 0 | Vehicle Identification Number | ||
| 46 | |
/SAPSLL/CUSCS | /SAPSLL/CUSCS | CHAR | 5 | 0 | Customs Code List Procedure | /SAPSLL/TCUCS | |
| 47 | |
/SAPSLL/FDACN | /SAPSLL/OBJEX | CHAR | 40 | 0 | FDA Registration Number | ||
| 48 | |
/SAPSLL/FDATS | /SAPSLL/TSTMP | DEC | 15 | 0 | FDA Date of Release |
 Foreign Keys
Foreign Keys
| |
Source Table | Source Column | Foreign Table | Foreign Column | Dependency Factor | Cardinality left | Cardinality right |
|---|---|---|---|---|---|---|---|
| 1 | /SAPSLL/CUHD_LOAD_0490_S | CUSCS_FDAPU | |
|
KEY | 1 | CN |
| 2 | /SAPSLL/CUHD_LOAD_0490_S | CUSEMP | |
|
KEY | 1 | CN |
| 3 | /SAPSLL/CUHD_LOAD_0490_S | CUSMAN | |
|
KEY | 1 | CN |
| 4 | /SAPSLL/CUHD_LOAD_0490_S | FDACP | |
|
REF | 1 | CN |
| 5 | /SAPSLL/CUHD_LOAD_0490_S | FDAUM | |
|
KEY | 1 | CN |
 History
History
| Last changed by/on | SAP | 20141106 |
| SAP Release Created in | 800 |